Page 51 - 2018食藥署年報(英文版)
P. 51
training programs) on the official webpage to promulgate policies and official directions as
references for applicants.
2. Schedule and related laws of GDP implementation
On February 18, 2016, the TFDA promulgated the implementation details, and schedules
of “Good Manufacturing Practice (GMP) Part III: Distribution.” Manufacturers and permit
holders of Western medications are expected to meet all regulations starting from 2019.
To achieve full-GDP implementation, the MOHW actively revises corresponding laws by
promulgating the drafts of Pharmaceutical Affair Act Article 53-1 and Article 92 amendments.
Such amendments have been promulgated under official Presidential Order on June 14, 2017.
The “Western Pharmaceuticals Good Distribution Practice Regulations” were then released on
December 28, 2017, as the GDP standard for Western drug dealers. Furthermore, to form a well-
established management system, the MOHW also stipulated the “Regulations for the Issuance
and Management of Western Pharmaceuticals Distribution Licenses and Certificates” to meet the
requirements for a subsequent application and approval issuance.
Outcomes and Benefits
By promoting and implementing global well-recognized GDP, ensuring drug safety and
improving drug distribution quality, 275 manufacturers and dealers of Western pharmaceuticals
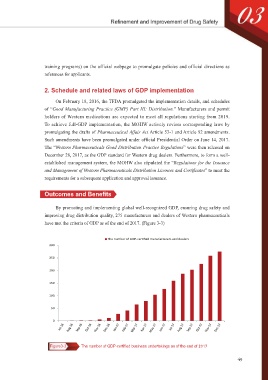
have met the criteria of GDP as of the end of 2017. (Figure 3-3)
The number of GDP-certified manufacturers and dealers
300
250
200
150
100
50
0
Figure3-3 The number of GDP-certified business undertakings as of the end of 2017
49