Page 47 - 2018食藥署年報(英文版)
P. 47
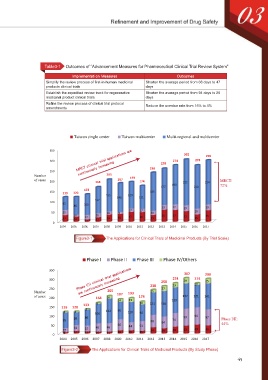
Table3-1 Outcomes of “Advancement Measures for Pharmaceutical Clinical Trial Review System”
Implementation Measures Outcomes
Simplify the review process of first-in-human medicinal Shorten the average period from 68 days to 47
products clinical trials days
Establish the expedited review track for regenerative Shorten the average period from 94 days to 26
medicinal product clinical trials days
Refine the review process of clinical trial protocol Reduce the overdue rate from 15% to 4%
amendments
Taiwan single center Taiwan multicenter Multi-regional and multicenter
MRCT clinical trial applications are 274 298
350 302
300 258 274
250 continuously increasing 238
Number 205
of cases 200 168 187 193 174 222 214 MRCT:
177 190 210 72%
133
150 119 120 185
155 129 111
127 140
100 62
86 100 23 27 29 29
17 17 29
50 25 16 9 20
10 20 47 58 57 51 55
32 24 22 21 34 38 46 33 35
0 11
2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 2017
Figure3-1 The Applications for Clinical Trials of Medicinal Products (By Trial Scale)
Phase I Phase II Phase III Phase IV/Others
Phase I/II clinical trial applications 274 274
350 302 298
300 258 29 10 25
250 are continuously increasing 238 27 23
Number 205 187 193 15
of cases 200 168 16 174 137 121 141
14 21 139
133 6 16 121 138
150 119 120 3
4 4 106 132 95 110 90
100 86 93 94 87
85 69 68 64 74 Phase I/II:
50 33 46 46 60 44 53 44%
22 32 18 18 34 29 38 43 49 45
0 8 14 12 10 11 15
2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 2017
Figure3-2 The Applications for Clinical Trials of Medicinal Products (By Study Phase)
45