Page 10 - 2018食藥署年報(英文版)
P. 10
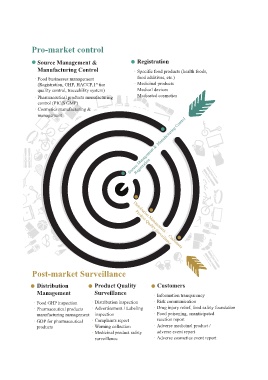
Pro-market control
Source Management & Registration
Manufacturing Control .Specific food products (health foods,
.Food businesses management food additives, etc.)
(Registration, GHP, HACCP,1 tier .Medicinal products
st
quality control, traceability system) Medical devices
.Pharmaceutical products manufacturing Medicated cosmetics
control (PIC/S GMP)
.Cosmetics manufacturing &
management
Soruce Management & Manufacturing Control
Registration
Product Quality Surveillance
Post-market Surveillance Distribution Management. Customers
Distribution Product Quality Customers
Management Surveillance .Information transparency
.Food GHP inspection .Distribution inspection .Risk communication
.Pharmaceutical products .Advertisement / Labeling .Drug injury relief, food safety foundation
manufacturing management inspection .Food poisoning, unanticipated
.GDP for pharmaceutical .Complaints report reaction report
products .Warning collection .Adverse medicinal product /
.Medicinal product safety adverse event report
surveillance .Adverse cosmetics event report