Page 96 - 2017食品藥物管理署年報(英文版)
P. 96
2017 Taiwan Food and Drug Administration Annual Report
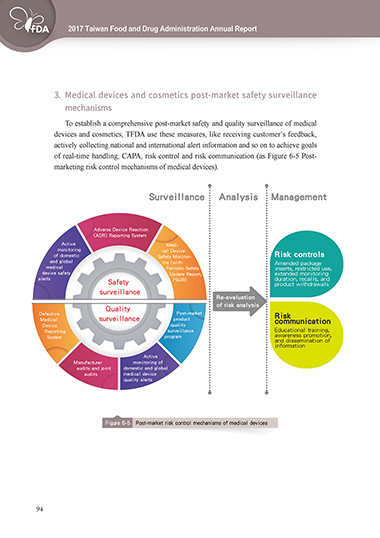
3. Medical devices and cosmetics post-market safety surveillance
mechanisms
To establish a comprehensive post-market safety and quality surveillance of medical
devices and cosmetics, TFDA use these measures, like receiving customer’s feedback,
actively collecting national and international alert information and so on to achieve goals
of real-time handling, CAPA, risk control and risk communication (as Figure 6-5 Post-
marketing risk control mechanisms of medical devices).
Surveillance Analysis Management
Adverse Device Reaction
(ADR) Reporting System
Active Medi-
monitoring cal Device
of domestic Safety Monitor- Risk controls
and global ing (with Amended package
medical Periodic Safety inserts, restricted use,
device safety Update Report, extended monitoring
alerts PSUR) duration, recalls, and
Safety product withdrawals
surveillance
Re-evaluation
of risk analysis
Quality
Defective Post-market Risk
Medical surveillance product communication
Device quality
Reporting surveillance Educational training,
System program awareness promotion,
and dissemination of
information
Active
Manufacturer monitoring of
audits and joint domestic and global
audits medical device
quality alerts
Figure 6-5 Post-market risk control mechanisms of medical devices
94