Page 91 - 2017食品藥物管理署年報(英文版)
P. 91
2017 Taiwan Food and Drug Administration Annual Report Chapter 6. Early Warning, Monitoring and Risk Management
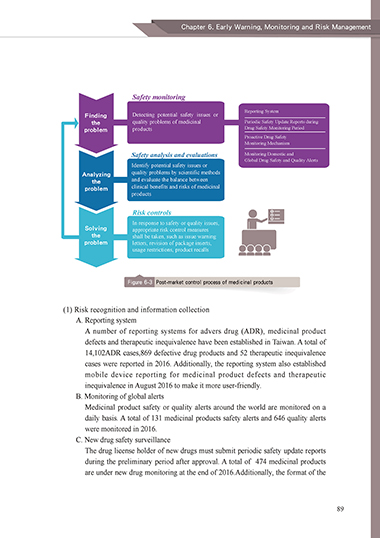
Safety monitoring
Reporting System
Finding Detecting potential safety issues or
the quality problems of medicinal Periodic Safety Update Reports during
problem products Drug Safety Monitoring Period
Proactive Drug Safety
Monitoring Mechanism
Safety analysis and evaluations Monitoring Domestic and
Global Drug Safety and Quality Alerts
Identify potential safety issues or
Analyzing quality problems by scientific methods
the and evaluate the balance between
problem clinical benefits and risks of medicinal
products
Risk controls
In response to safety or quality issues,
Solving appropriate risk control measures
the shall be taken, such as issue warning
problem letters, revision of package inserts,
usage restrictions, product recalls
Figure 6-3 Post-market control process of medicinal products
(1) Risk recognition and information collection
A. Reporting system
A number of reporting systems for advers drug (ADR), medicinal product
defects and therapeutic inequivalence have been established in Taiwan. A total of
14,102ADR cases,869 defective drug products and 52 therapeutic inequivalence
cases were reported in 2016. Additionally, the reporting system also established
mobile device reporting for medicinal product defects and therapeutic
inequivalence in August 2016 to make it more user-friendly.
B. Monitoring of global alerts
Medicinal product safety or quality alerts around the world are monitored on a
daily basis. A total of 131 medicinal products safety alerts and 646 quality alerts
were monitored in 2016.
C. New drug safety surveillance
The drug license holder of new drugs must submit periodic safety update reports
during the preliminary period after approval. A total of 474 medicinal products
are under new drug monitoring at the end of 2016.Additionally, the format of the
89