
86
2014 Annual Report
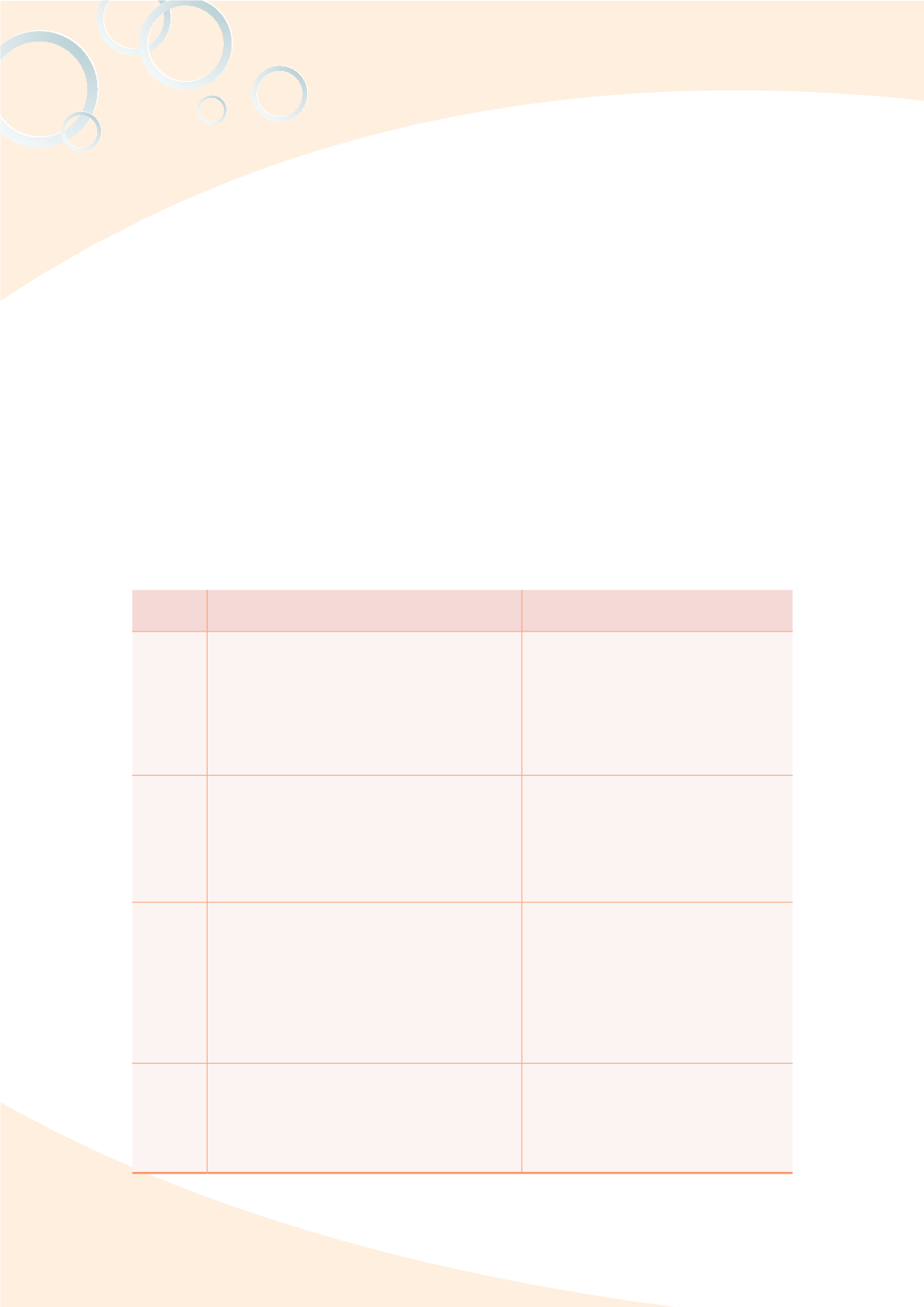
;HISL 9LSL]HU[ ZWLJPÄJH[PVU KYHM[Z VM UHUV TH[LYPHS WYVK\J[Z WYVWVZLK I` [OL ;-+(
Type of
product
5HTL VM THUHNLTLU[ ZWLJPÄJH[PVU
Test method (draft) and effectiveness
Nano
drugs
1. Checklist for chemical, manufacturing
and control and management Technology
Information of Nano drugs (proposed)
2. Technology information list for new liposome
drugs and agents, new dosage, and unit
amount attached as required for the inspection
and registration of generic drugs (proposed)
3. CMC screening standards for liposome drugs
Established methods to measure the
particle diameters of drug ingredients
after nano processed and practically
applied to the discussion on the
grinding conditions of nano Chinese
herb medicine
Nano
medical
devices
7VPU[Z [V JVUZPKLY MVY PKLU[PÄJH[PVU VM
application of nanotechnology in medical
devices
2. Draft template of EP/STED for dental
composites
3. Precautions for biological evaluation of
medical devices containing nano-materials
1. Nano anti-bacteria safety evaluation
guidelines (draft)
5HUV KLU[HS ÄSSPUN YLZPU ZHML[`
evaluation guidelines (draft)
Nano
cosmetics
1. Rules governing the requirements for
submitting technology, safety, and stability
data for reviews of cosmetics containing
nano-materials
2. Recommendations for attaching the table
of physical and chemical properties and the
table of biological safety evaluation with the
submission of cosmetics containing nano-
ingredients
Establish analytical methods of the
Zinc oxide and Titanium dioxide
nanoparticles in cosmetics, and using
the above method to characterize the
commercial compact powder
Nano
food
1. Guidance on the safety assessment of
nano-scale food)
2. Nano-scale food packaging materials safety
assessment method (draft)
1. Test methods for particle diameters of
nano calcium and nano pearl powder
(draft)
2. Food utensils, containers, and package
test methods- Tests of plastics
contained with nano silver (draft)
for the education and management of nano-scale food to build the dialogues and
communication channel for the industry, the government, academia, and the research
institutions in order to achieve the goals of exchanges and building consensus.
(2) In 2013, there were 11 proposals of management and evaluation assessment (including
drafts) and recommendations were proposed which cover current updates for
sectors of global nanotechnology such as new information of product management,
application and safety concerns.
2. The Potential for Bio-Nanotechnology to Grow
As nano technology advances rapidly, the TFDA continuously enacts effective test
methods for nano related products. In 2013, test methods completed and the
effectiveness are shown below (Table 9-7).



















