
28
2014 Annual Report
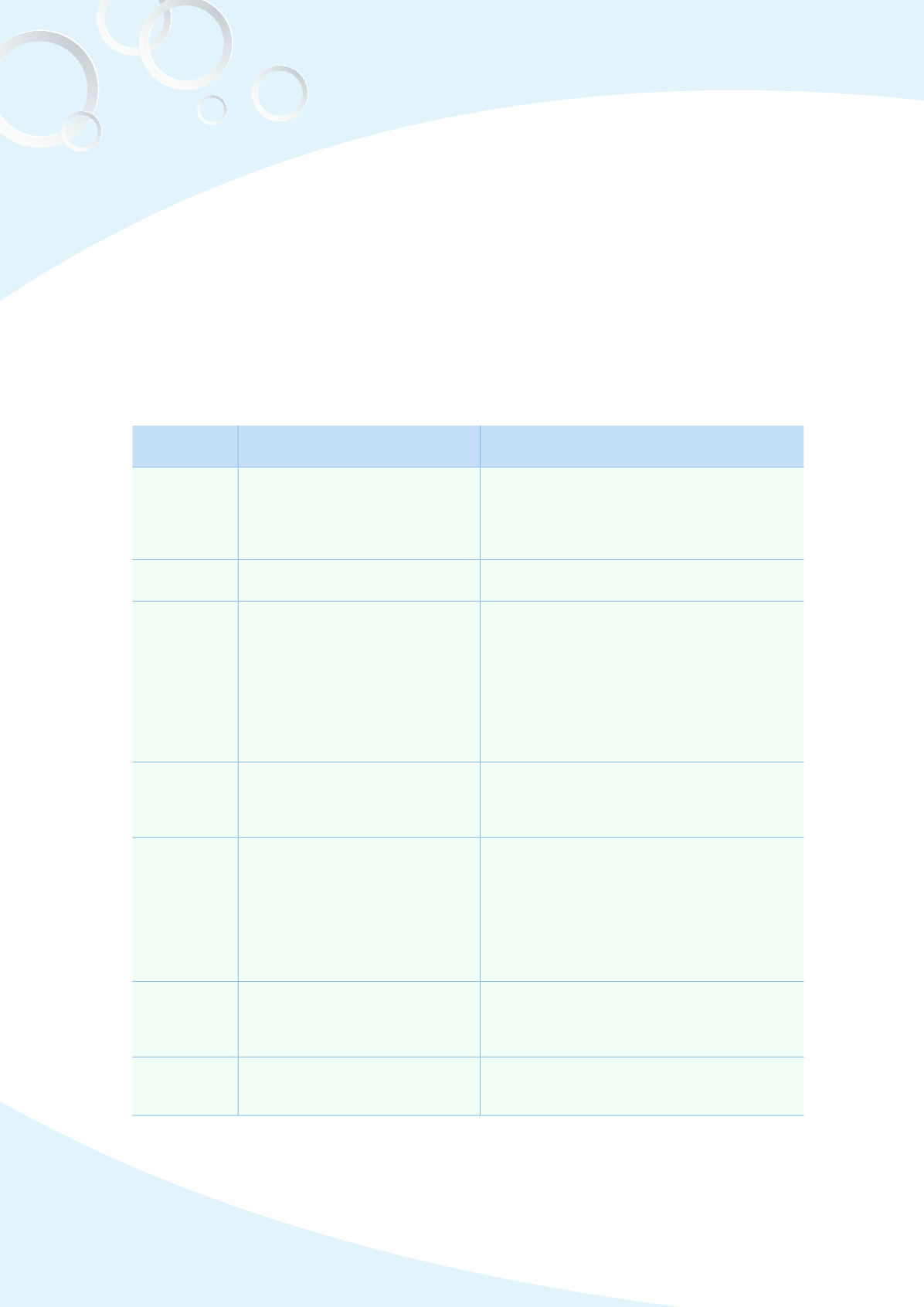
Table 3-1 Revisions and additions to regulations governing the management of
pharmaceutical affairs, 2013
Publication
date
Regulation/specification
Summary
January 31 Regulations for Bioavailability and
Bioequivalence Studies
Amendments of article 15, 17 and 21, which
included the cross-over design of BA/BE
studies, the data exclusion criteria, and the
description of using basket/paddle methods
during dissolution tests
February 23 Guidance for Investigational New
Drug Applications
Any new drug clinical trial application must
fulfill the current requirement.
March 11
Revision for the set up standard
for new pharmaceutical
manufacturing factory, Amendment
for Standards for Medicament
Factory Establishments
and Pharmaceutical Good
Manufacturing Practice
Regulations
Combine Part 3 for
“
Good Manufacturing
Practices for Pharmaceuticals
”
and Part
4 as
“
Good Manufacturing Practices for
Medical Devices
”
of the set up standard for
new pharmaceutical manufacturing factory,
and then transferred without modification
to under the Part 2 and 3 sections for
“
the
Pharmaceutical Good Manufacturing Practice
Regulations
”
April 17 Guideline for Review and Approval
of Botanical Drug Products
The application and approval guidelines for
the botanical new drug products. A separate
regulation was issued to meet the distinctive
properties of the botanical drug products
April 18
Guideline for Review and Approval
of new chemical entities which
have been approved for over 10
years in the 10 advanced countries
This guideline lists the requirement of technical
document for marketing approval of drugs
which have been approved in the 10 advanced
countries for over 10 years, including new
chemical entity drug in Taiwan. There are
plenty clinical information for those drugs, the
publicly available information is accepted to
substitute for partial submission document
May 22
Announcement the Part 2, titled
as
“
Guide to Good Manufacturing
Practice for Medicinal Products
(Part II)
”
Provide GMP reference for manufacturers of
Active Pharmaceutical Ingredients, in Chinese
and English for the
“
Good Drug Manufacturers
Guidelines
”
May 29
Guideline for the Nonclinical
Pharmacology/Toxicology Studies
for Medicinal Products Applications
Removing outdated standards for physiological
values of laboratory animals and specific
pathogen-free experimental animals
Section 1 Product Regulations and Registration of Medicinal Products
Policy and Outcome
1. Comprehensive Standardized Regulation



















