
112
2014 Annual Report
regulations, management systems and inspection standards have already gained
international recognition and our domestic pharmaceutical quality is keeping pace with
that of advanced countries.
4
.
4LTILYZOPW )LULÄ[Z
After becoming an official member of PIC/S, Taiwan will not only be listed on the
PIC/S Rapid Alert System; our international image has also been significantly
LUOHUJLK HUK V\Y .47 PUZWLJ[PVU YLWVY[ JHU IL YLJVNUPaLK NSVIHSS` PU HKKP[PVU [V
avoiding duplicated inspections, and leveraging the inspection resources. Domestic
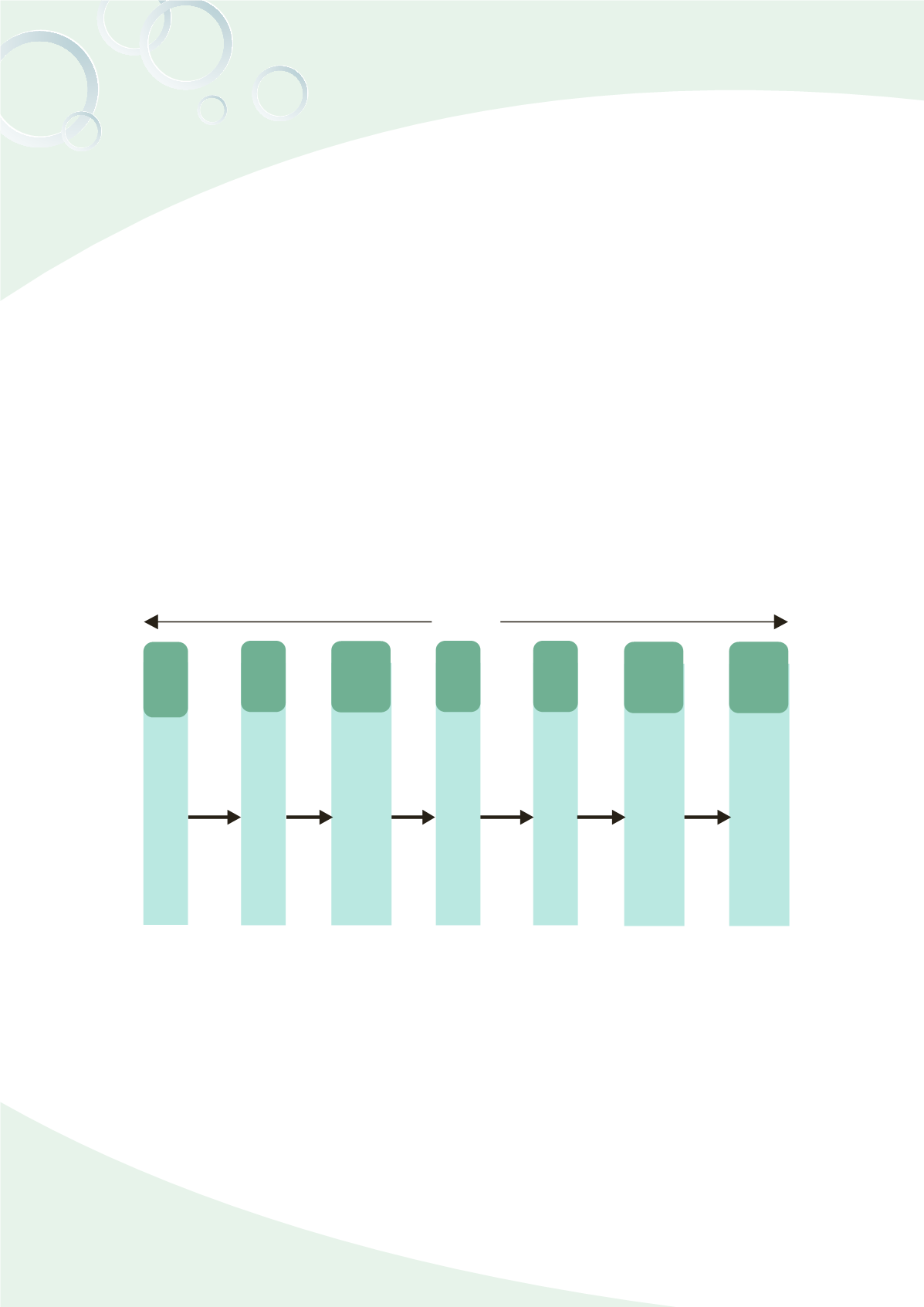
June
2010
2010
July
2011
June
2012
Sep.
2012
Oct.
2012
Jan.
2013
Submitted for PIC/S
membership
PIC/S conducted dossier
review
To have face to face discussion
with the PIC/S rapporteur about
the dossier review.
TFDA submitted the corrective
action report for the assessment.
On-site assessment by the
PIC/S delegates.
The PIC/S committee accepted
TFDA’s membership application.
TFDA has become the official
participating authority of PIC/S,
ahead of Japan and South Korea.
2.5 years
Fig. 12-1 PIC/S Admission timing



















