
57
Food and Drug Administration
[V LUZ\YL JVTWSPHUJL ^P[O ]VS\U[HY` JVZTL[PJZ .47 HUK [V JVUMVYT [V PU[LYUH[PVUHS
norms and standards. Referring to the international cosmetics management policy, we
propose the
“
Cosmetics Product Notification Portal
”
>P[O [OL L_LTW[PVU VM [OL WYL
market approval & registration when implementing this new system, we aim to shorten
time to the market and improve competitiveness of the products.
Policy and Outcome
1. Source Control
Since 2008, more and more manufacturers have applied and been approved for
]VS\U[HY` JVZTL[PJ .47 H\[OLU[PJH[PVU HZ ZOV^U PU -PN
0U [OL M\[\YL ^L
^PSS JVU[PU\L [V WYVTV[L .47 [V LUOHUJL [OL PU[LYUH[PVUHS JVTWL[P[P]LULZZ VM [OL
cosmetics in our country.
,Z[HISPZOTLU[ VM *VZTL[PJZ 7YVK\J[ 5V[PÄJH[PVU 7VY[HS
In order to implement the
“
Cosmetics Product Notification Portal
”
, we referred to
ZWLJPÄJH[PVUZ VM UV[PÄJH[PVU WVY[HS ^VYSK^PKL JVUZPKLYLK [OL ULLKZ VM V\Y JV\U[Y`
and industry, and designed the
“
Cosmetics Product Notification Portal
”
(shown in
Table 5-3).
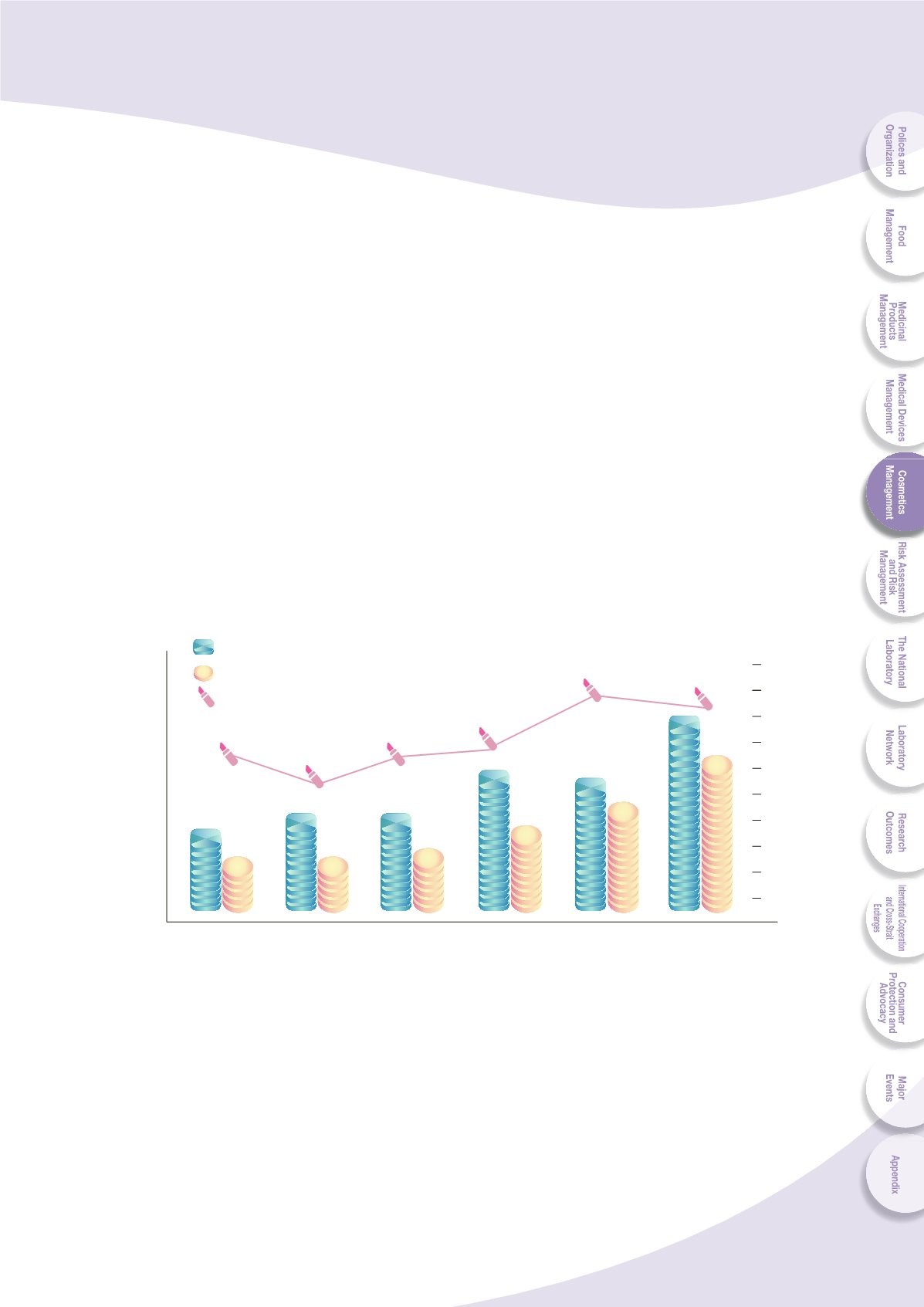
Fig. 5-3 List of applications and approval for cosmetic product good
manufacturing practices (GMP) from 2008 to 2013
0
40
50
30
10
20
60
70
80
90
55.6
9
5
0
10
5
15
20
25
30
2008
2009
2010
2011
2012
2013
5
11
11
6
16
9
15
12
24
18
45.5
54.5
56.3
80
75
Approval rate (%)
Approval
Applications



















