
56
2014 Annual Report
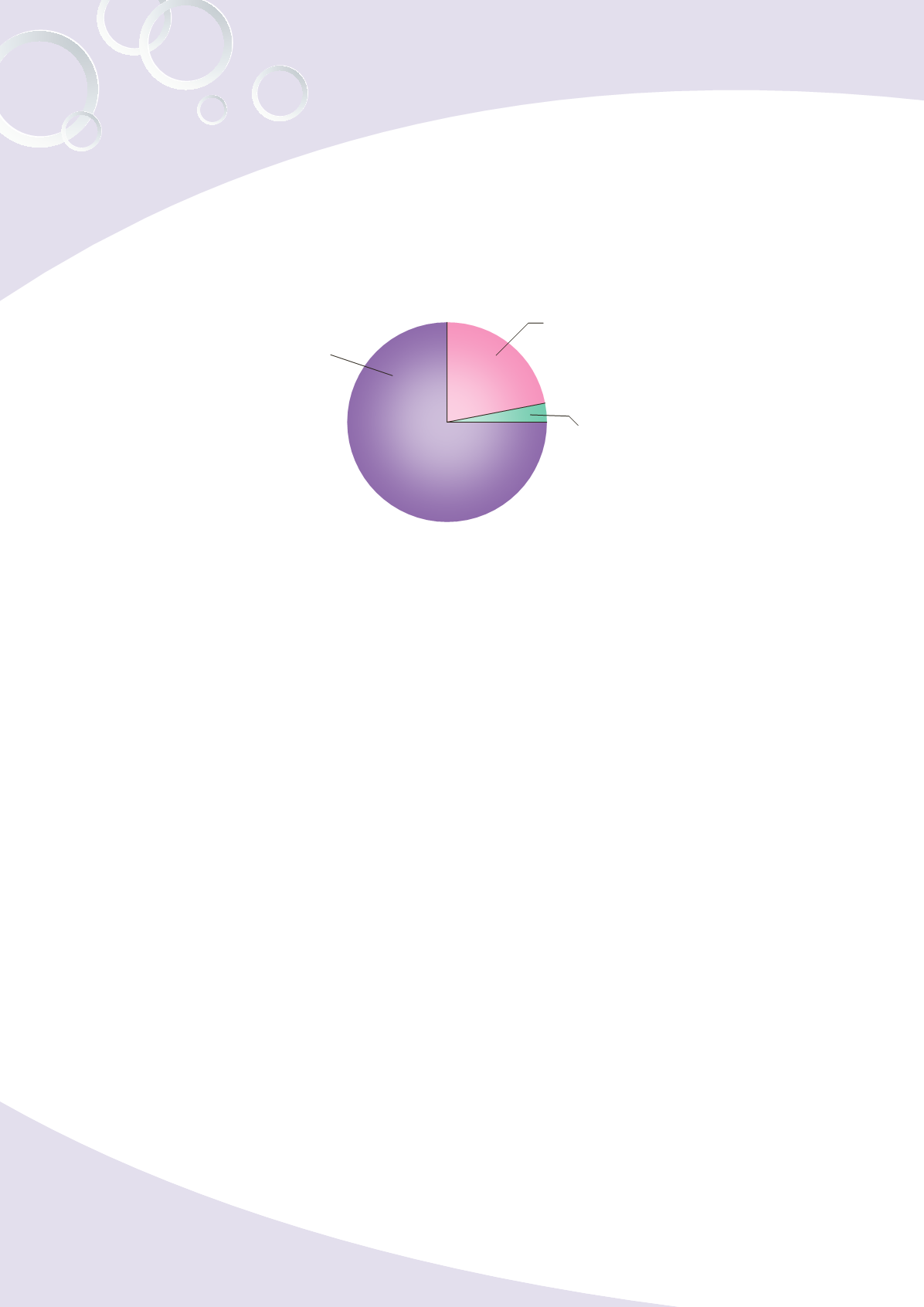
b. Until year 2013, total number of medicated cosmetic licenses issued by the Bureau
were 27,078, including a total number of 5,927 and 21,151 licenses for domestic
products and imported products, respectively (as shown in Fig. 5-2)
(2) Advertising examination for cosmetics
a. In order to unify the examination standards for the review authorities, we set up
“
Cosmetics Advertising Act and Examination Manual
”
,
“
Guideline for Cosmetics
Advertising
”
and
“
Rules for Application of Drugs and Cosmetics Advertising
”
, for
vendors to follow as guidelines of advertising examination.
b. We regularly hold meetings with health authorities to deal with advertising
examination and improve quality of examination, in order to achieve examination
consistency.
c. We hold training programs on reviews to strengthen the principle of consensus. In
addition, the Cosmetics Advisory Councils, which have already been established,
study special cases and draft the complete advertisement regulations for cosmetics;
meanwhile, also assist TFDA to amend
“
Enumeration of Expressions that are
Appropriate or Inappropriate to be Claimed for Cosmetics
”
.
d. In 2013, a total of 1,261 cosmetic advertisements were examined, and 1,192 of
them were approved.
Section 2 Cosmetics Source Control
Status
Since 2008, we have worked alongside with the Industrial Development Bureau, Ministry
of Economic Affairs; to promote the program designed for cosmetics manufacturers
Fig. 5-2 Total number of medicated cosmetic licenses granted until year 2013
Imported licenses 20,296
75%
Domestic licenses 5,927
22%
Imported from
Mainland China 855
3%



















