
50
2014 Annual Report
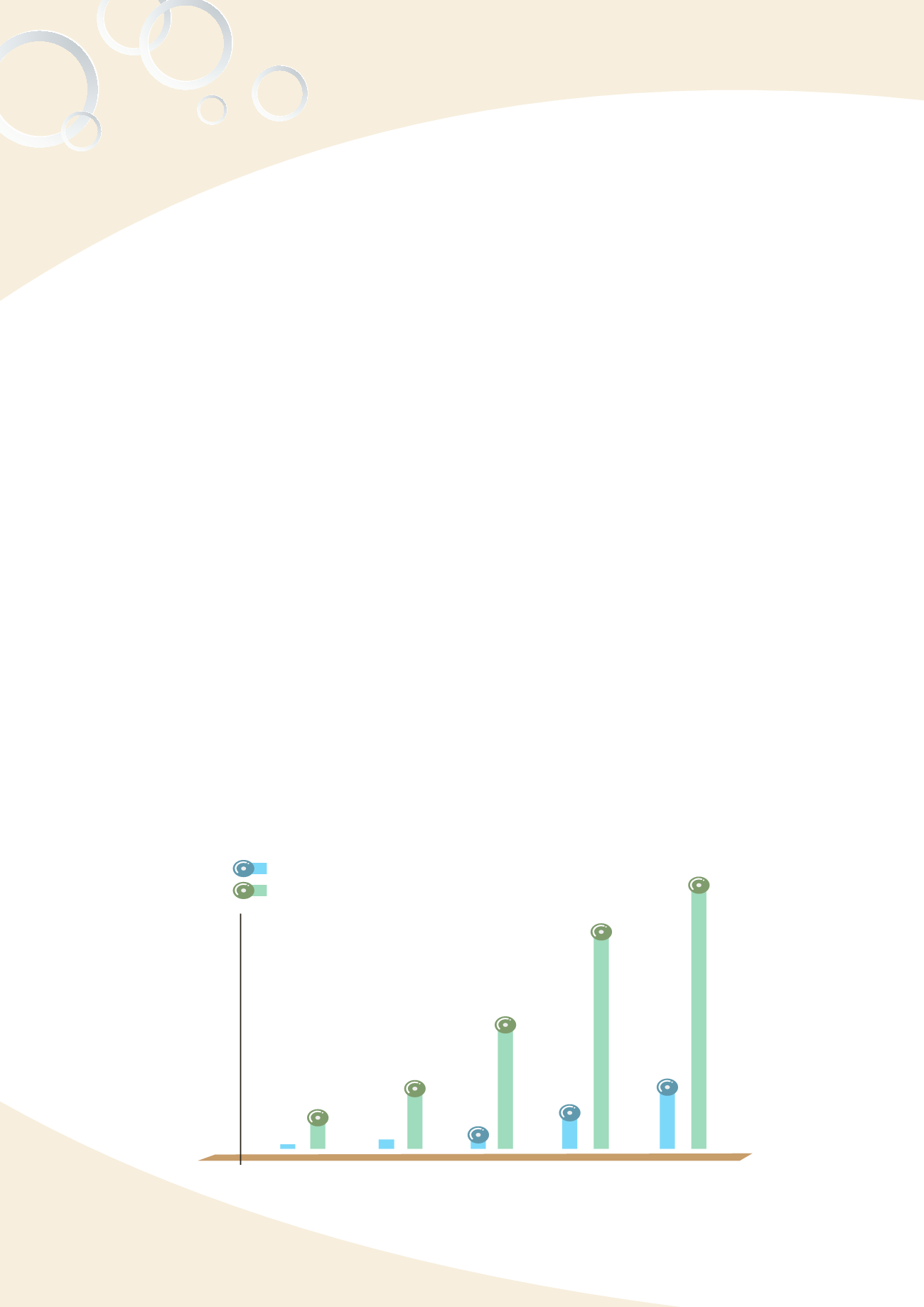
Fig. 4-4 Defective products/adverse reactions reporting for medical devices
2009
Adverse event reation notification
Product defect notification
2011
2010
2012
2013
1300
1100
900
700
500
300
100
-100
30
157
54
366
70
748
285
1368
372
1634
Section 4 Medical Device Safety Management
Status
To improve safety surveillance, TFDA has set up the medical device adverse event/
defective product reporting system, continues to conduct medical devices post-marketing
surveillance, proactively monitors global medical device safety alerts, promotes the Good
Distribution Practice, and disseminate regulatory policies.
Policy and Outcome
1. Strengthening Medical Device Post-Marketing Safety Monitoring
(1) Monitor global medical devices safety alerts proactively
In 2013, a total of 3,737 medical device safety alerts were processed, including 1,985
product warnings and 1,752 recall requests. The TFDA flagged 127 safety warnings
which were related to domestic safety and asked for extra attention.
(2) Join the National Competent Authority Report exchange program (NCAR).
The TFDA is a member of the NCAR of the International Medical Device Regulators
Forum (IMDRF). We receive the recall notifications and safety warnings from the member
states. In 2013, 399 global warning reports were received.
(3) Increase reports of medical device adverse reactions and defective products
The TFDA encouraged manufacturers and the public to report medical device adverse
reactions and defective products via the medical device reporting system. From 2009
to 2013, reports of adverse reactions increased from 30 to 372 and defective products
increased from 157 to 1634 (Fig.4-4).



















