Page 56 - 2020Taiwan Food and Drug Administration Annual Report
P. 56
$FKLHYHPHQWV DQG %HQH¿WVFKLHYHPHQWV DQG %HQH¿WV
$
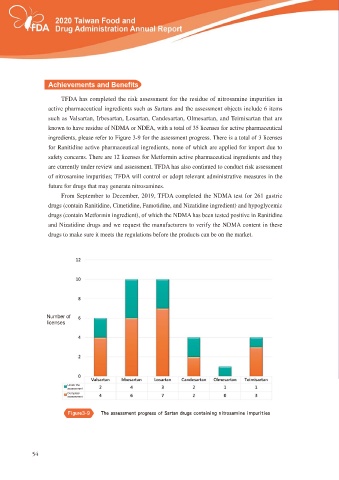
TFDA has completed the risk assessment for the residue of nitrosamine impurities in
active pharmaceutical ingredients such as Sartans and the assessment objects include 6 items
such as Valsartan, Irbesartan, Losartan, Candesartan, Olmesartan, and Teimisartan that are
known to have residue of NDMA or NDEA, with a total of 35 licenses for active pharmaceutical
ingredients, please refer to Figure 3-9 for the assessment progress. There is a total of 3 licenses
for Ranitidine active pharmaceutical ingredients, none of which are applied for import due to
safety concerns. There are 12 licenses for Metformin active pharmaceutical ingredients and they
are currently under review and assessment. TFDA has also continued to conduct risk assessment
of nitrosamine impurities; TFDA will control or adopt relevant administrative measures in the
future for drugs that may generate nitrosamines.
From September to December, 2019, TFDA completed the NDMA test for 261 gastric
drugs (contain Ranitidine, Cimetidine, Famotidine, and Nizatidine ingredient) and hypoglycemic
drugs (contain Metformin ingredient), of which the NDMA has been tested positive in Ranitidine
and Nizatidine drugs and we request the manufacturers to verify the NDMA content in these
drugs to make sure it meets the regulations before the products can be on the market.
Number of
licenses
Under the
assessment
Complete
assessment
'JHVSF cc5IF BTTFTTNFOU QSPHSFTT PG 4BSUBO ESVHT DPOUBJOJOH OJUSPTBNJOF JNQVSJUJFT
54