Page 43 - 2020Taiwan Food and Drug Administration Annual Report
P. 43
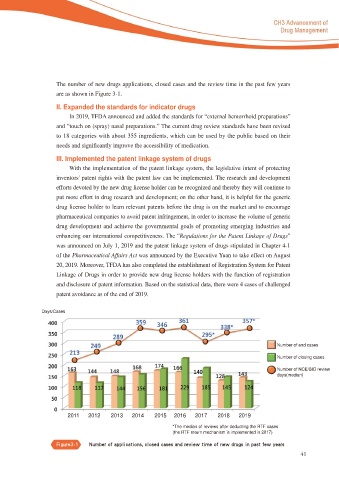
The number of new drugs applications, closed cases and the review time in the past few years
are as shown in Figure 3-1.
II. Expanded the standards for indicator drugs
In 2019, TFDA announced and added the standards for “external hemorrhoid preparations”
and “touch on (spray) nasal preparations.” The current drug review standards have been revised
to 18 categories with about 355 ingredients, which can be used by the public based on their
QHHGV DQG VLJQL¿FDQWO\ LPSURYH WKH DFFHVVLELOLW\ RI PHGLFDWLRQ
III. Implemented the patent linkage system of drugs
With the implementation of the patent linkage system, the legislative intent of protecting
inventors' patent rights with the patent law can be implemented. The research and development
efforts devoted by the new drug license holder can be recognized and thereby they will continue to
put more effort in drug research and development; on the other hand, it is helpful for the generic
drug license holder to learn relevant patents before the drug is on the market and to encourage
pharmaceutical companies to avoid patent infringement, in order to increase the volume of generic
drug development and achieve the governmental goals of promoting emerging industries and
enhancing our international competitiveness. The “Regulations for the Patent Linkage of Drugs”
was announced on July 1, 2019 and the patent linkage system of drugs stipulated in Chapter 4-1
of the Pharmaceutical Affairs Act was announced by the Executive Yuan to take effect on August
20, 2019. Moreover, TFDA has also completed the establishment of Registration System for Patent
Linkage of Drugs in order to provide new drug license holders with the function of registration
and disclosure of patent information. Based on the statistical data, there were 4 cases of challenged
patent avoidance as of the end of 2019.
Days/Cases
Number of end cases
Number of closing cases
Number of NCE/BIO review
days(median)
2011 2012 2013 2014 2015 2016 2017 2018 2019
*The median of reviews after deducting the RTF cases
(the RTF return mechanism is implemented in 2017)
'JHVSF cc/VNCFS PG BQQMJDBUJPOT DMPTFE DBTFT BOE SFWJFX UJNF PG OFX ESVHT JO QBTU GFX ZFBST
41