Page 100 - 2019食藥署年報(英文版)
P. 100
$BQBDJUZ UFTU JOUFSOBUJPOBM DPMMBCPSBUJWF TUVEZ
0SHBOJ[FS 3FTFBSDI SFTVMU
3FTFBSDI OBNF
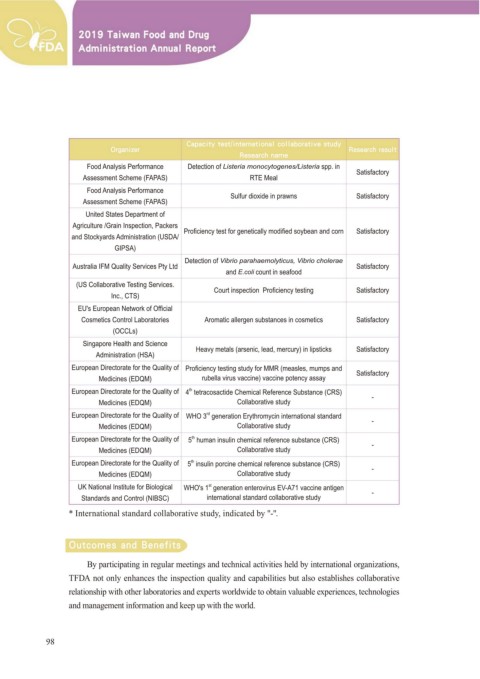
Food Analysis Performance Detection of Listeria monocytogenes/Listeria spp. in
*NQMFNFOUBUJPO .FBTVSFT Satisfactory
Assessment Scheme (FAPAS) RTE Meal
Food Analysis Performance
Assessment Scheme (FAPAS) Sulfur dioxide in prawns Satisfactory
United States Department of
$JULFXOWXUH *UDLQ ,QVSHFWLRQ 3DFNHUV
DQG 6WRFN\DUGV $GPLQLVWUDWLRQ 86'$ 3UR¿FLHQF\ WHVW IRU JHQHWLFDOO\ PRGL¿HG VR\EHDQ DQG FRUQ Satisfactory
*,36$
Detection of Vibrio parahaemolyticus, Vibrio cholerae
$XVWUDOLD ,)0 4XDOLW\ 6HUYLFHV 3W\ /WG Satisfactory
and E.coli count in seafood
86 &ROODERUDWLYH 7HVWLQJ 6HUYLFHV &RXUW LQVSHFWLRQ 3UR¿FLHQF\ WHVWLQJ Satisfactory
,QF &76
(8 V (XURSHDQ 1HWZRUN RI 2I¿FLDO
&RVPHWLFV &RQWURO /DERUDWRULHV $URPDWLF DOOHUJHQ VXEVWDQFHV LQ FRVPHWLFV Satisfactory
2&&/V
6LQJDSRUH +HDOWK DQG 6FLHQFH +HDY\ PHWDOV DUVHQLF OHDG PHUFXU\ LQ OLSVWLFNV
$GPLQLVWUDWLRQ +6$ Satisfactory
European Directorate for the Quality of 3UR¿FLHQF\ WHVWLQJ VWXG\ IRU 005 PHDVOHV PXPSV DQG Satisfactory
Medicines (EDQM) UXEHOOD YLUXV YDFFLQH YDFFLQH SRWHQF\ DVVD\
European Directorate for the Quality of 4 WHWUDFRVDFWLGH &KHPLFDO 5HIHUHQFH 6XEVWDQFH &56
th
Medicines (EDQM) &ROODERUDWLYH VWXG\
European Directorate for the Quality of :+2 JHQHUDWLRQ (U\WKURP\FLQ LQWHUQDWLRQDO VWDQGDUG
rd
Medicines (EDQM) &ROODERUDWLYH VWXG\
th
European Directorate for the Quality of 5 KXPDQ LQVXOLQ FKHPLFDO UHIHUHQFH VXEVWDQFH &56
Medicines (EDQM) &ROODERUDWLYH VWXG\
th
European Directorate for the Quality of 5 LQVXOLQ SRUFLQH FKHPLFDO UHIHUHQFH VXEVWDQFH &56
Medicines (EDQM) &ROODERUDWLYH VWXG\
8. 1DWLRQDO ,QVWLWXWH IRU %LRORJLFDO :+2 V JHQHUDWLRQ HQWHURYLUXV (9 $ YDFFLQH DQWLJHQ
st
6WDQGDUGV DQG &RQWURO 1,%6& LQWHUQDWLRQDO VWDQGDUG FROODERUDWLYH VWXG\
* International standard collaborative study, indicated by "-".
0
0VUDPNFT BOE #FOFGJUTVUDPNFT BOE #FOFGJUT
By participating in regular meetings and technical activities held by international organizations,
TFDA not only enhances the inspection quality and capabilities but also establishes collaborative
relationship with other laboratories and experts worldwide to obtain valuable experiences, technologies
and management information and keep up with the world.
98