Page 99 - 2018食藥署年報(英文版)
P. 99
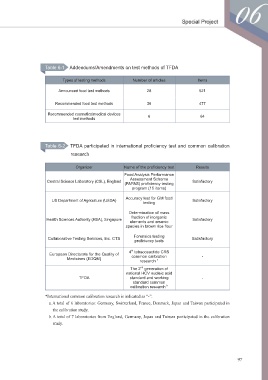
Table 6-1 Addendums/Amendments on test methods of TFDA
Types of testing methods Number of articles Items
Announced food test methods 28 521
Recommended food test methods 36 477
Recommended cosmetics/medical devices 6 64
test methods
Table 6-2 TFDA participated in international proficiency test and common calibration
research
Organizer Name of the proficiency test Results
Food Analysis Performance
Assessment Scheme
Central Science Laboratory (CSL), England Satisfactory
(FAPAS) proficiency testing
program (15 items)
Accuracy test for GM food
US Department of Agriculture (USDA) Satisfactory
testing
Determination of mass
fraction of inorganic
Health Sciences Authority (HSA), Singapore Satisfactory
elements and arsenic
species in brown rice flour
Forensics testing
Collaborative Testing Services, Inc. CTS Satisfactory
proficiency tests
th
4 tetracosactide CRS
European Directorate for the Quality of common calibration -
Medicines (EDQM)
research a
nd
The 2 generation of
national HCV nucleic acid
TFDA standard and working -
standard common
calibration research b
*International common calibration research is indicated as “-”.
a.A total of 6 laboratories: Germany, Switzerland, France, Denmark, Japan and Taiwan participated in
the calibration study.
b.A total of 7 laboratories from England, Germany, Japan and Taiwan participated in the calibration
study.
97