Page 62 - 2018食藥署年報(英文版)
P. 62
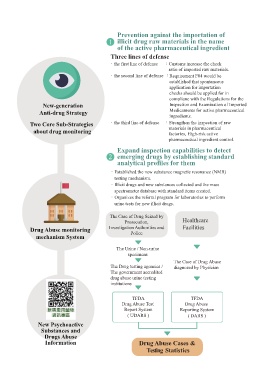
Prevention against the importation of
1 illicit drug raw materials in the name
of the active pharmaceutical ingredient
Three lines of defense
.the first line of defense :Customs increase the check
ratio of imported raw materials.
.the second line of defense :Requirement F04 would be
established that spontaneous
application for importation
checks should be applied for in
compliane with the Regulations for the
New-generation Inspection and Examination of Imported
Anti-drug Strategy Medicaments for active pharmaceutical
ingredients.
Two Core Sub-Strategies .the third line of defense :Strengthen the inspection of raw
about drug monitoring materials in pharmaceutical
factories, High-risk active
pharmaceutical ingredient control.
Expand inspection capabilities to detect
2 emerging drugs by establishing standard
analytical profiles for them
.Established the new substance magnetic resonance (NMR)
testing mechanism.
.Illicit drugs and new substances collected and the mass
spectrometer database with standard items created.
.Organizes the referral program for laboratories to perform
urine tests for new illicit drugs.
The Case of Drug Seized by
Prosecution, Healthcare
Drug Abuse monitoring Investigation Authorities and Facilities
mechanism System Police
The Urine / Non-urine
specimens
The Case of Drug Abuse
The Drug testing agencies / diagnosed by Physician
The government accredited
drug abuse urine testing
institutions
TFDA TFDA
Drug Abuse Test Drug Abuse
Report System Reporting System
( UDARS ) ( DARS )
New Psychoactive
Substances and
Drugs Abuse
Information Drug Abuse Cases &
Testing Statistics