Page 16 - Taiwan Food and Drug Administration 2016 Annual Report
P. 16
Taiwan Food and Drug Adminstration
Section 2. Organization Framework
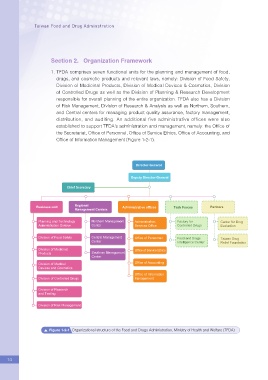
1. TFDA comprises seven functional units for the planning and management of food,
drugs, and cosmetic products and relevant laws, namely: Division of Food Safety,
Division of Medicinal Products, Division of Medical Devices & Cosmetics, Division
of Controlled Drugs as well as the Division of Planning & Research Development
responsible for overall planning of the entire organization. TFDA also has a Division
of Risk Management, Division of Research & Analysis as well as Northern, Southern,
and Central centers for managing product quality assurance, factory management,
distribution, and auditing. An additional five administrative offices were also
established to support TFDA's administration and management, namely: the Of?ce of
the Secretariat, Of?ce of Personnel, Of?ce of Service Ethics, Of?ce of Accounting, and
Of?ce of Information Management (Figure 1-2-1).
Director-General
Deputy Director-General
Chief Secretary
Regional
Business unit Administrative offices Task Forces Partners
Management Centers
Planning and Technology Northern Management Administration Factory for Center for Drug
Administration Division Center Services Office Controlled Drugs Evaluation
Division of Food Safety Central Management Office of Personnel Food and Drugs Taiwan Drug
Center Intelligence Center Relief Foundation
Division of Medicinal Office of Service Ethics
Products Southern Management
Center
Division of Medical Office of Accounting
Devices and Cosmetics
Office of Information
Division of Controlled Drugs Management
Division of Research
and Testing
Division of Risk Management
Figure 1-2-1 Organizational structure of the Food and Drugs Administration, Ministry of Health and Welfare (TFDA)
14