
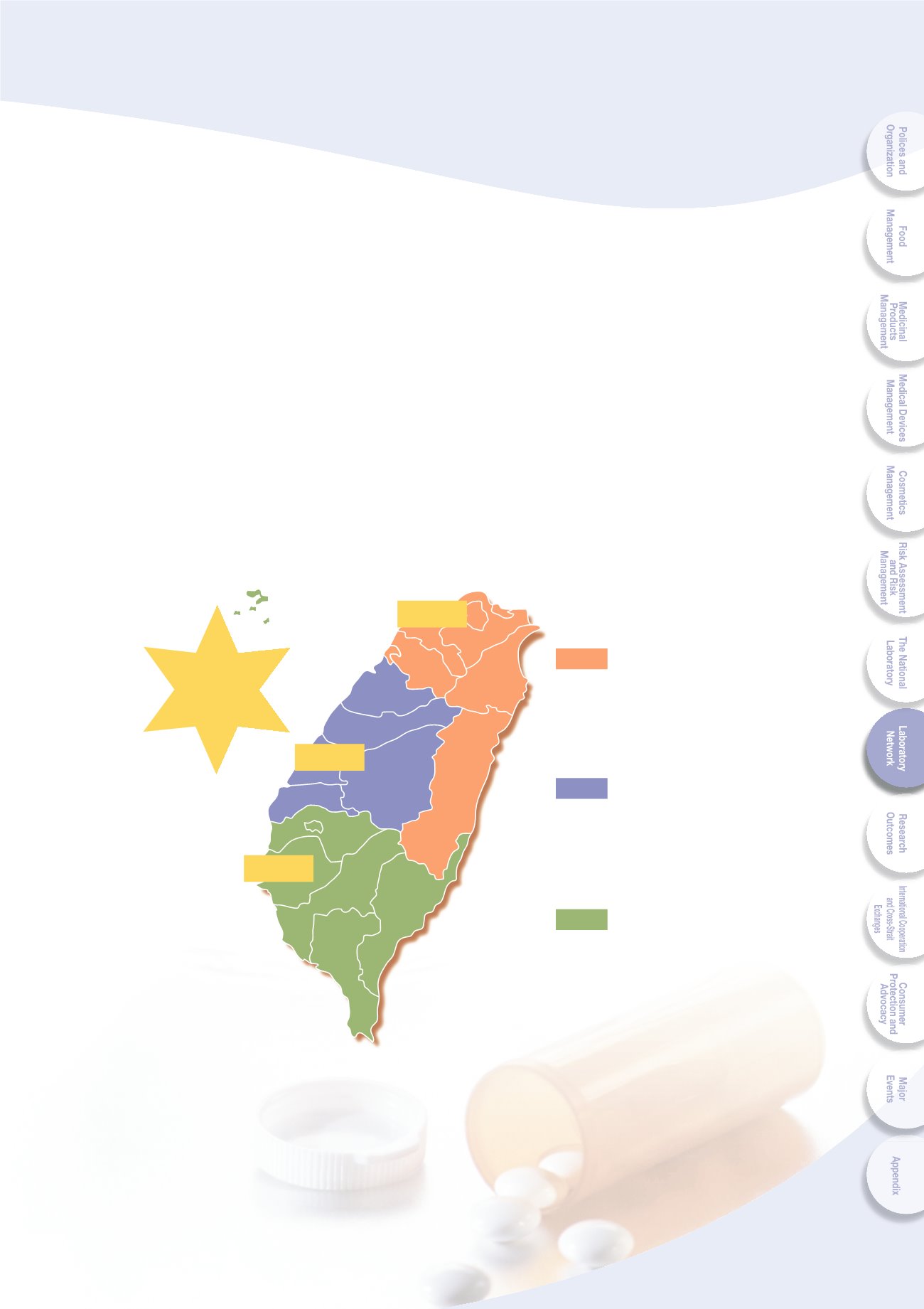
73
Food and Drug Administration
Central FDA : 8
Local labs: 10
Private labs:
67
Central FDA : 1
Local labs: 7
Private labs:
35
Central FDA : 1
Local labs: 5
Private labs:
17
North region
Central region
Southern region
85 labs
23 labs
151
labs
43 labs
Fig. 8-2 Numbers and distribution status of TFDA accredited laboratories
Section 2 Laboratory Accreditation and Management
Status
TFDA has been actively organizing free of charge accreditation for private laboratories in
order to (1) effectively utilize testing resources of private laboratories, (2) ensure the quality
and credibility of the commissioned tests, and (3) expand the testing forces. Currently
TFDA accreditation scope includes (1) food, (2) drug and cosmetics, (3) drug abuse urine
testing and (4) non-clinical GLP testing, and provides laboratories with testing capacities
and credibility. Besides, those laboratories can meet emergency testing demands.
Accreditation status is as shown in Fig. 8-2, the number of accredited items is 1,192.



















