
43
Food and Drug Administration
ୋ
z
ᔼᐕኜҿ၍ଣ
z
4
ᎇഹ߅Ҧ˚อ˜ମʘ೯࢝eᔼᐕ
ڭ
߅ҦʷʘცӋdᒕɝϼϋʷʘΚዚdᔼᐕኜ
ҿପุϓ
މ
Ң௰Ոᆑɢٙ͛Ҧପุfࠦ࿁lzᔼᐕኜҿପุٙ
ݺ
ഖၾۜሯ၍ଣd੶ሜ
˸ऊ൬٫
ڭ
ᚐ
މ
ࣨːdʱй
ج
၍ଣყʷe͛ପ๕᎘છ၍eɪ̹
ۃ
Ҫᗫeɪ̹
ܝ
္
ຖeᖹਠʿପۜஷ༩၍ଣʿਖ਼ุፔ༔Ⴞኬd
ܔ
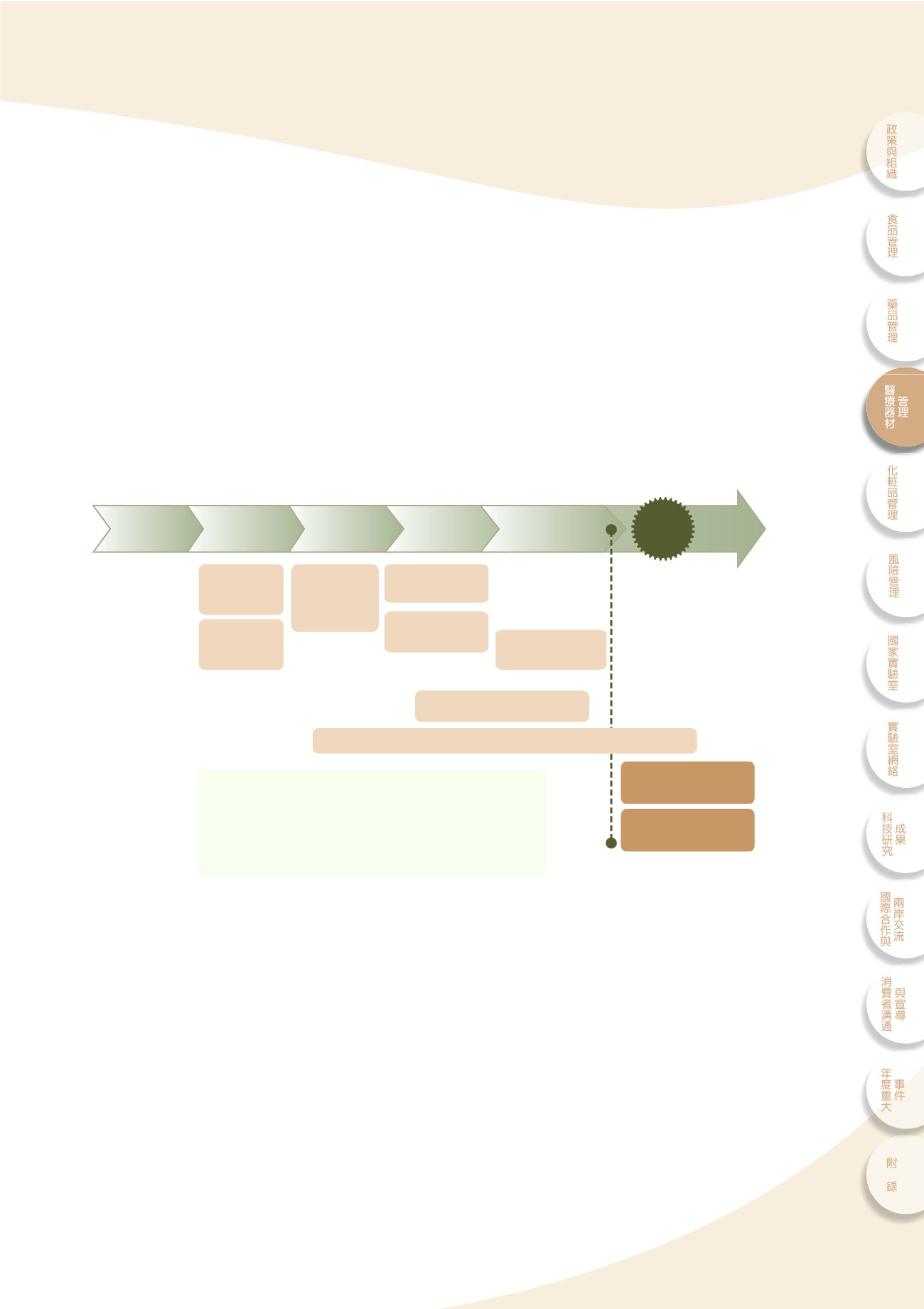
ͭᔼᐕኜҿΌ͛նಂ
Total product life
cycle
ۜሯ၍ଣ݁ഄྡ
4-1
dϞࣖછ၍ᔼᐕኜҿʘτΌeࣖঐʿۜሯdΝࣛ੭ਗҢ
͛Ҧᔼᖹପุ೯࢝d௴ऊ൬٫eุ٫ʿִ݁ɧᙊʘ҅ࠦf
ୋɓືcᔼᐕኜҿ
ج
ᅺʿପۜᄲ
ݟ
ତ
ر
ᔼᐕኜҿ
جމ
৷
ࠅܓ
Ӌ၍ଣٙପุd
ܔ
ͭீeϞࣖଟʿΥଣٙ
ج
ᐑྤd
ڭ
ღɛԴ͜τΌʿ
ڮ
ආପุ೯࢝f
މ
ᐵପᔼᐕኜҿɪ̹ᄲ
ݟ
ࣛʿɪ̹
ۃ
Չۜሯ
τΌϞࣖʘҪᗫdྼ݄ᑗґ
ۃ
༊eᑗґ༊᜕ʿପۜᏨ᜕ᅺഃ
ݟ
᜕೮াʘ၍ଣධ
ͦᄲ
ݟ
f̤০࿁Ң௴อ೯ʘ͡ሗࣩdԶุ٫
ج
ፔ༔ਕʿਖ਼ࣩႾኬd݊
пପุ೯࢝ٙࠠ
ࠅ
лਿf
݁ഄၾϓ
؈
ɓe
ج
ᅺყሜձʷ
102
ϋᔼᐕኜҿ
ج
ʿᗫʮѓᄣ
ࠈࡌ
8
ධ
ڌ
4-1
f
ᔼᐕᚐცӋ
ਿᓾӺ
ɓছāਖ਼ࣩ
ፔ༔Ⴞኬ
ᔼҿ
ݟ
᜕೮াᏨ
ྼ᜕܃ᇍ
ᑗґ༊᜕
ݟ
ࣨ
GCP
ݟ
᜕೮া
Approval
ā
Listing
ᑗґ༊᜕
ࠇ
ᄲ
ݟ
TFDA/IRB
ପۜ᙮к֛
͛ପ๕᎘၍ଣ
ପۜண
ࠇ
ࡡۨක೯
ᑗґ
ۃ
᜕ᗇ
ᑗґ༊᜕ ɪ̹͡ሗ
ඎପ
ɪ̹
ɪ̹
ۃ
Ҫᗫ
ɪ̹
ܝ
္છ
ᔼᐕኜҿਖ਼։ࡰፔᙄ
Ⴁிᅀۜሯӻ୕ᇆ
ݟ
GMP
ʔԄۜā
ʔԄԫ
ஷ
జ
ADR
τΌ္ൖʿᙆৃႎණ
GVP
GLP
GLP
GCP
IRB
GMP
ADR
GVP
j
Good Laboratory Practice
ྼ᜕܃ᎴԄЪᇍ
j
Good Tissue Practice
ɛ
ߤ
ଡ଼ᔌᎴԄЪᇍ
j
Good Clinical Practice
ᑗґ༊᜕ᎴԄᇍ
j
Institutional Review Board
ɛ༊᜕։ࡰึ
j
Good Manufacturing Practuce
ᎴԄႡிᇍ
j
Adverse Drug/Device Reactions
ᖹ
ي
ʔԄˀᏐ
j
Good Vigilance Practices
ɪ̹
ܝ
ᎴԄτΌ္ൖᇍ
GLP/GTP
ྡ
4-1
cҢᔼᐕኜҿΌ͛նಂ၍ଣӻ



















